Ronen Zangi and Danilo Roccatano
Nano Lett. 2016, DOI: 10.1021/acs.nanolett.6b00460
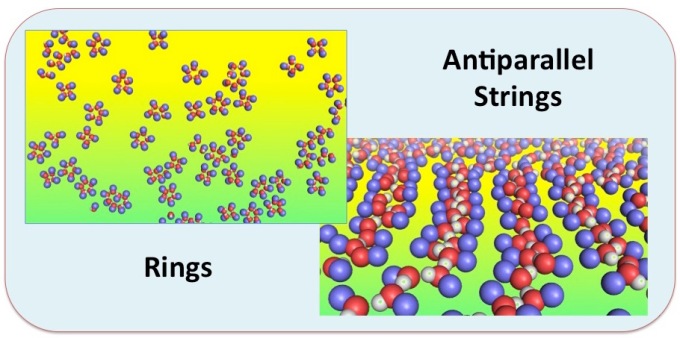
Structural order emerging in the liquid state necessitates a critical degree of anisotropy of the molecules. For example, liquid crystals and Langmuir monolayers require rod/disc-shaped and long chain amphiphilic molecules, respectively, to break the isotropic symmetry of liquids. In this paper, we present results from molecular dynamics simulations demonstrating that in two-dimensional liquids, a significantly smaller degree of anisotropy is sufficient to allow structural organization. In fact, the condensed phase of the smallest amphiphilic molecule, methanol, confined between two or adsorbed on, graphene sheets form a monolayer characterized by long chains of molecules. Intra-chain interactions are dominated by hydrogen bonds, whereas inter-chain interactions are dispersive. Upon a decrease in density toward a gas-like state, these strings are transformed into rings. The two-dimensional liquid phase of methanol undergoes another transition upon cooling; in this case, the order-disorder transition is characterized by a low-temperature phase in which the hydrogen bond dipoles of neighboring strings adopt anti-parallel orientation.
